Research Overview
Project I: Develop toolkits of biomaterials for precise and flexible control of CAR-T cell phenotype that excels in the local environment.
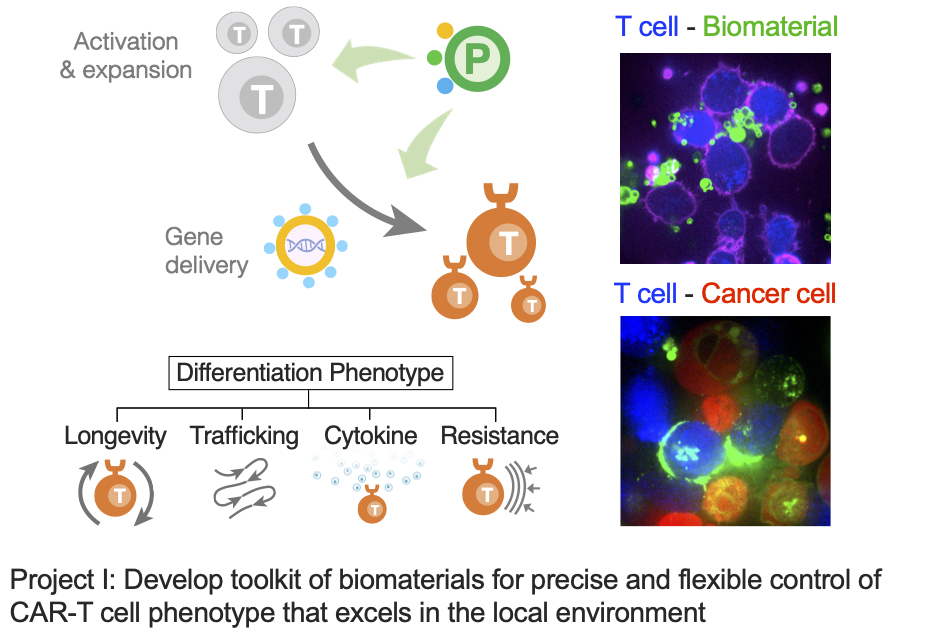
Chimeric antigen receptor (CAR) T cell therapy, as a successful example of immune cell engineering, is revolutionizing the clinical treatment of B cell malignancies. However, it still faces major challenges in treating a broad population of patients and other types of cancer. CAR-T cell manufacturing typically involves ex vivo activation of human T cells for expansion and CAR gene engineering. Manufacturing optimizations have mainly focused on the cell product quantity, but little attention has been given to their quality in terms of memory or effector phenotype. Memory cells have better proliferative features and are equipped to persist over time, while effector cells feature higher metabolic activity and are more prevalent in local tissues. Precise control of these phenotypes can be essential for optimal anti-tumor efficacy in different tumor environments. In this project, we develop biomaterial platforms that stimulate T cells and deliver CAR genes to generate cell products with ideal phenotypes for different disease environments.
Project II: Construct implantable recharge stations and mod shops for T cells through precision biomaterials with multi-functionalities
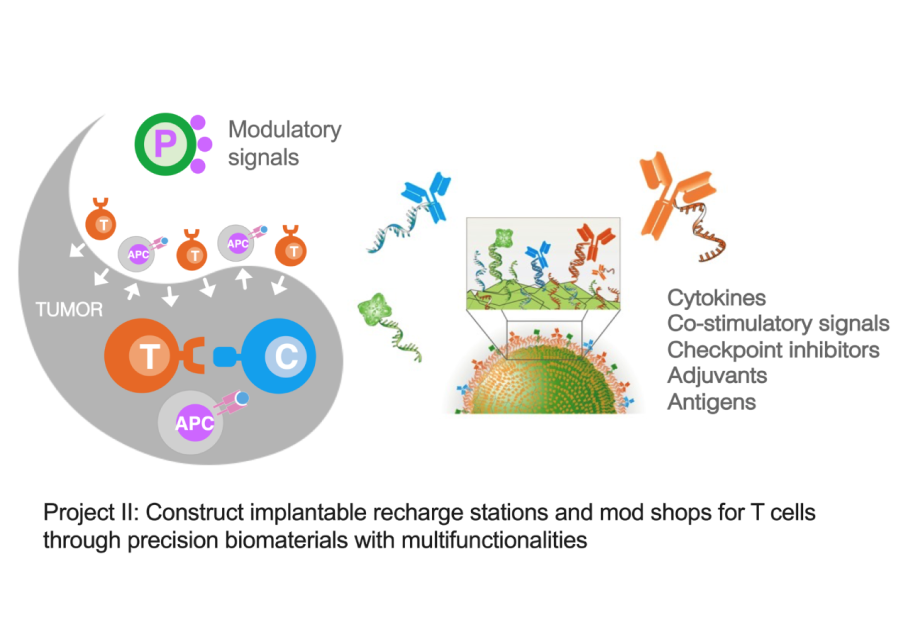
An emerging narrative from many studies is that the optimal anti-tumor T cell phenotype is biphasic. During the ex vivo expansion and priming phase of immunotherapy, the maintenance of memory cell phenotype with high proliferation capacity is key. Conversely, at the site of the tumor in vivo, especially for solid tumors, the effector phenotype with robust metabolic activity for anabolic synthesis is required to overcome the suppressive environment and exert their cytotoxicity. Therefore, we aim to use pharmacological interventions, for example, administrating antigens, co-stimulatory ligands, and checkpoint inhibitors, to modulate phenotypic features after in vivo infusion of T cells. These immunomodulatory molecules can be delivered via biocompatible materials to extend their half-life in vivo and to enable the spatio-temporal control of immune cells. Furthermore, surface display of signals on synthetic surfaces and their quantitative control can better mimic natural cell-cell interfaces to precisely program cell phenotypes for optimal efficacy.
Project III: Build in vitro tumor models with spatial precision through synthetic engineering of materials and cells to study T cell infiltration
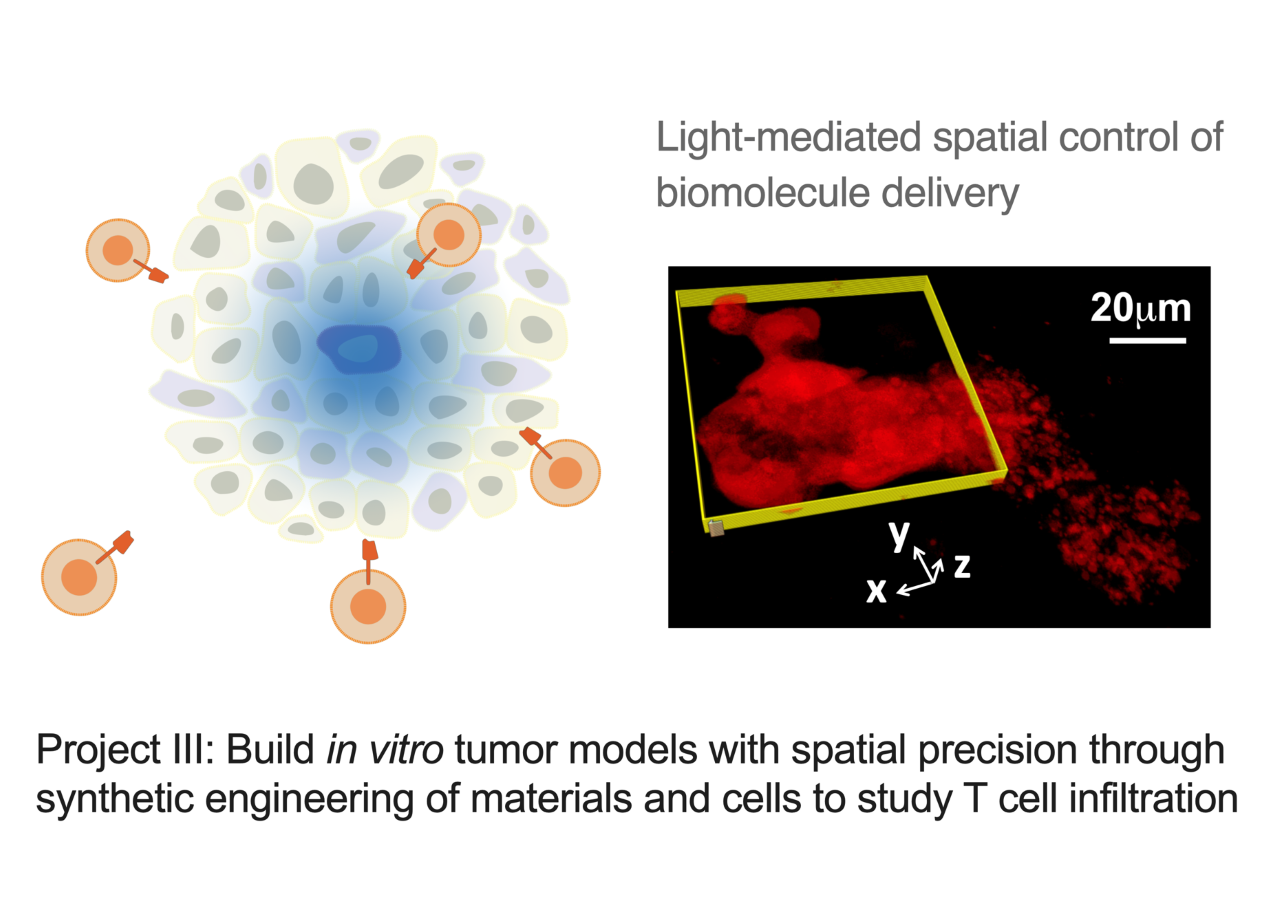
Cytokines and chemokines (CK) form essential communication language among tissue-resident cells and immune cells in systems immunology. Deciphering this language is key for developing effective immune cell therapy that can turn “cold” tumor (non-T cell inflamed) into “hot” tumor (T cell inflamed) in difficult situations. CK naturally form gradient signals to guide immune cell migration. However, it is challenging to generate in vivo models with this precision and to quantify the dynamic cell migration process. Therefore, in vitro tumor spheroids with live-cell imaging can bring advances for high-throughput and quantitative screening of CK that facilitate T cell homing. In this project, we aim to develop 3D-cultured organoids with a radial-gradient pattern of CK distribution to enable the real-time and quantitative T cell migration study. We use a combinatorial approach of materials engineering and synthetic biology to spatially program CK secretion within the tumor organoid.


