Human Factors Engineering of Medical Devices
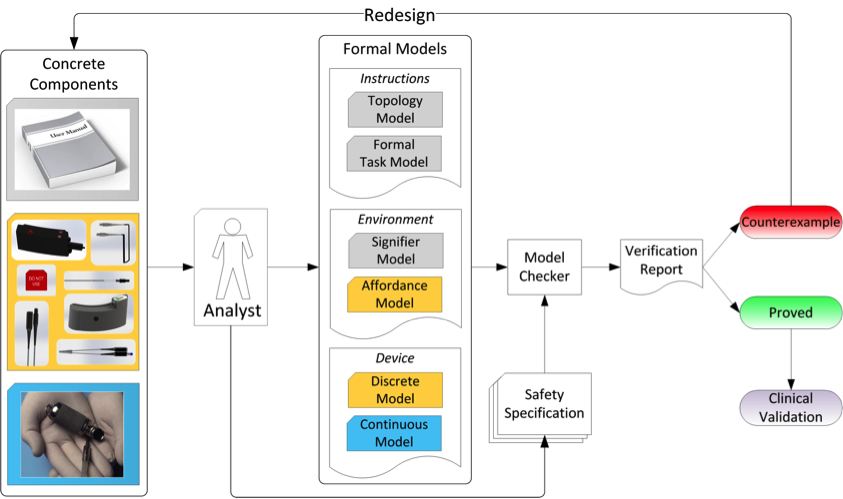
A conceptual representation of our model-based approach to medical device usability analysis.
The human factors division of the BioCirc lab focuses on leveraging formal methods from computer science to augment human factors testing. We are currently developing a computational framework to support model-based analysis of user interfaces (including user manuals) within medical device systems as a proactive effort to help manufacturers of novel, lifesaving medical devices uncover and correct usability problems. The figure above shows a conceptual representation of the framework under development. Formal, mathematical models of the user manual, the device interface and the device plant (such as a heart pump) can be encoded, composed into a system, and analyzed exhaustively by a model checker to verify requirements that specify usability parameters. This work aims to improve user manual evaluation methods, galvanize formal human factors testing, and decrease occurrence rates of use-related adverse events, ultimately saving lives.

