Overview
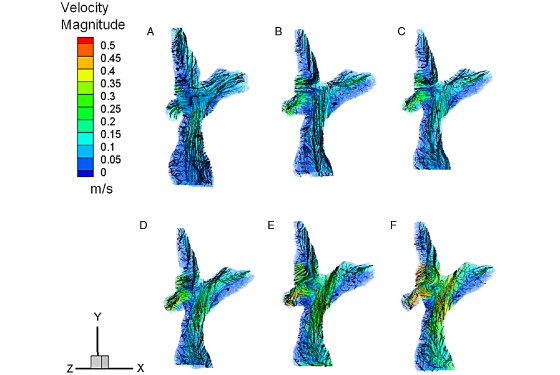
Citation: S.G. Chopski, O.M. Rangus, C.S. Fox, W.B. Moskowitz, and A.L. Throckmorton. "Stereo-PIV Measurements of a Patient-Specific Fontan Physiology using Novel Pressure Augmentation Stents." Artificial Organs 2014, In press.
Congestive heart failure (CHF) is a progressive and debilitating disease that affects tens of millions of children and adults worldwide. In the U.S., more than 7 million people suffer from heart failure, and more than 500,000 new cases are diagnosed per year. This costs the healthcare industry tens of billions of dollars annually, and only 2500 donor hearts are available each year. Thousands are registered awaiting a donor heart; many of those patients who are on the waiting list die. Thus, many patients would benefit from temporary or long-term mechanical circulatory support (MCS). This explains, in part, why the use of TAHs and ventricular assist devices (VADs) is on the rise. This field has greatly benefited from technological advances that have enabled a paradigm shift from the use of pulsatile VADs to smaller rotary or continuous flow pumps. The latest TAHs and rotary blood pumps incorporate design innovation related mostly to the power drive systems, such as the use of magnetic bearings, as opposed to traditional mechanical (fluid or pivot) bearings or positive-displacement polyurethane-based pumps with valves. These design advances and the findings of the REMATCH and INTREPID clinical trials demonstrate that patients who are ineligible for transplantation, or are in need of a bridge-to-transplant, derive substantial survival and quality of life benefits from long-term MCS. While these findings are encouraging, there are many design limitations associated with currently approved devices and those under development. These limitations include the high propensity for thrombosis, bulkiness of the pumps, percutaneous drivelines leading to increased infection risks, size restrictions for younger or smaller patients, fixed impeller designs, invasive implantation, and more. To address these limitations of current technology, the BioCirc Laboratory at Drexel seeks to improve the treatment strategies and therapeutic options for patients suffering from heart disease by developing unique features for inclusion in the design of blood pumps and to develop entirely new designs of blood pumps for patients with single ventricle or biventricular circulations as a bridge-to-transplant, bridge-to-recovery, or destination therapy.
The new medical devices, as developed by the BioCirc Lab, have optimal hydraulic performance, lower manufacturing costs, lower incidence of blood trauma and thrombosis, properties of rapid exchangeability, and fewer moving parts. These blood pumps will support those patients in desperate need of circulatory assistance in the systemic or pulmonary circulation. The developmental procedure of these assist devices requires careful consideration of biophysical factors, such as their internal fluid regions, hydraulic performance, cardiophysiology and pathophysiology, biocompatibility and materials, hemocompatibility, electronics and controllers, motor and bearings, cannulae designs, and failure and reliability evaluations. Evaluative and design techniques include a multi-tiered approach with numerical simulations of steady and transient flow phenomena, 3-D reconstruction of patient-specific physiologies from MRI data to virtually and experimentally examine interactive fluid dynamics between patient anatomy and blood pumps, prototype manufacturing, in vitro hydraulic testing, hemolysis experiments, particle image velocimetry MRI flow measurements, and animal testing.

