Chapter 1. Primer on Ceramic Biomaterials in Orthopedics
Steven M. Kurtz, PhD.
Jump to a Section
1.1 Introduction
1.2 Ceramics Basics.
1.3 Ceramics Biomaterials for Total Joint Replacement
1.4 The Origins of Ceramics in Hip Replacement
1.5 Summary
1.6 Acknowledgements
1.7 References
1.1 Introduction
Ceramic materials are found everywhere in nature, whether created geothermally, as in gemstones, or grown biologically, like the calcium phosphate minerals that strengthen bone. Man-made ceramics such as pottery were developed in ancient times and are among the most durable artifacts of human technology; they can be traced back for thousands of years. Indeed, the origin of “ceramics” can be traced back to ancient Greek word for pottery. This is a very broad field of materials science, even if we restrict our attention to man-made ceramics.
Engineering ceramics, a subcategory of ceramics, are designed specifically in modern times for elevated temperature and high strength. There are many examples of engineering ceramics in use today, such as engine components or cutting tools like drill bits. Some types of engineering ceramics are widely used today as implantable biomaterials. The focus for this website will be the biomedical application of engineering ceramics, specifically in the medical field of orthopedics. An example of a spherical cup and femoral head component used to replace the hip socket are illustrated in Figure 1.1.
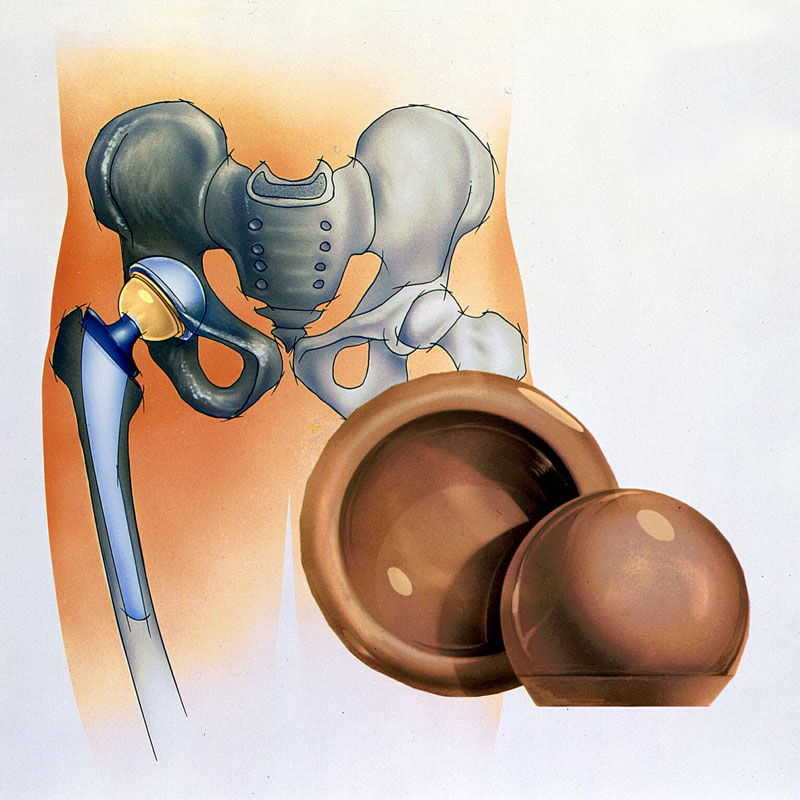
Figure 1.1. Examples of spherical components used to reconstruct the hip socket in a total hip replacement procedure. The female cup component used in reconstruction of the hip socket is called a liner, and the ball component replaces the femoral head.
The ceramic materials used in orthopedics today are formed by fusing or sintering a ceramic powder of microscopic grains of alumina (aluminum oxide, Al2O3) and/or zirconia (zirconium dioxide, ZrO2) into a consolidated head or liner, like those depicted in Figure 1.1. Ceramic components are now widely accepted around the world for bearing articulations for total hip replacements (THRs). In the United States, ceramic heads are used in over 40% of THRs [1], whereas in other countries such as the United Kingdom, ceramic bearings are even more popular [2]. Historically, alumina ceramics were first used as both femoral heads and acetabular components starting in the 1970s [3-5]. In 2003, a new generation of alumina matrix composites was clinically introduced with higher strength and fracture toughness than alumina [6]. Today, alumina matrix composites are gradually replacing alumina as the clinical material of choice for ceramic bearings.
Before we begin to delve deeply into the use of ceramic materials in medicine, it is important to begin with some basics that will help explain the clinical results that will be discussed in subsequent chapters on this website. Accordingly, for the remainder of this chapter we briefly outline some of the basic structure and property characteristics of ceramics and briefly recount the clinical history of ceramics in orthopedics. We also provide an introductory overview of the three specific types of ceramic materials relevant to hip replacement, namely alumina, zirconia, and alumina matrix composites. Readers interested in a more detailed treatment of these basic subjects and history of ceramics may wish to consult recent reviews published in the literature [6, 7]. For those who are already familiar with the basics, you may wish to skip ahead to Chapter 2 in which we discuss the design, manufacture, and reliability of ceramic implants.
^ Return to Top
1.2 Ceramic Basics
Ceramics are nonmetallic, inorganic solid compounds. If we restrict our attention to alumina (Al2O3) and zirconia (ZrO2) in their pure or ideal state, these compounds consist of either aluminum (Al) or zirconium (Zr) chemically bonded to oxygen (O). In some orthopedic circles, zirconia is referred to as “oxidized zirconium.” Although the terminology varies in the orthopedic literature, depending upon context and the author’s background: zirconia, zirconium oxide, and oxidized zirconium; all refer to the same compound, denoted in chemical shorthand as ZrO2.
Both alumina and zirconia are organized in dense, tightly packed repeating units known as crystals. The oxygen bonds in alumina and zirconia are extremely strong chemically speaking, and among the most difficult to break. Thus the crystalline structure helps to explain the hardness, strength, and chemical resistance of these ceramics. The crystalline structure also explains their inherent brittleness and intolerance of geometric flaws and stress concentrations. This limitation is now well understood and accounted for. Over the past forty years, there have been focused efforts to improve the resistance of ceramics to brittle fracture, which can be overcome by engineering design and reliability, which will be covered in Chapter 2, as well as by the principles of materials science and ceramic engineering, as discussed further below.
We have already mentioned how modern ceramic hip components are produced by sintering microscopic powders of pure alumina and/or zirconia. In the absence of design considerations, the strength of a ceramic component depends upon the purity and size of the granular powder particles, as well as upon the powder composition (i.e., alumina vs. zirconia). The sintered microstructure of alumina is shown in Figure 1.2.

Figure 1.2. A representative example of sintered alumina microstructure, in which the average grain size is 2 μm or less.
For orthopedic ceramics, the inherent fracture resistance is partially related to the size and distribution of internal flaws or defects, which are usually associated with poor sintering or with large grains. Thus, ensuring nearly full density and reducing the grain size of alumina ceramics over the past 40 years has resulted in an overall improvement in its fracture strength (Table 1.1).
Table 1.1.Various properties of alumina and zirconia ceramics used in the total hip reconstruction [8-10].
| Property | Alumina in 1970s | Alumina in the 1980s | Alumina in the 1990s | Zirconia | Alumina Matrix composite |
| Bending strength (MPa) | > 450 | > 500 | > 550 | > 900 | > 1000 |
| Fracture Toughness (MPa.m1/2) | 4 | 4 | 4 | 8 | 5.7 |
| Vickers hardness (0.1 kg) | 1800 | 1900 | 2000 | 1250 | 1975 |
| Grain size (micron) | 4.5 | 3.2 | 1.8 | < 0.5 | < 1.5 (Alumina Matrix) |
| Young's Modulus (GPa) | 380 | 380 | 380 | 210 | 350 |
Despite brittle characteristics, ceramic components enjoy several outstanding tribological properties, including their hardness (Table 1.1), which contributes to wear- and scratch-resistance. Ceramic surfaces are also more hydrophilic than the metal surfaces of a femoral head, as illustrated in Figure 1.3. The improved wettability of ceramics contributes to lower friction than metal when articulating in an artificial hip [11].

Figure 1.3.Ceramic surfaces are more hydrophilic than the metal surfaces of a femoral head, shown by the water droplets in the pictures.
^ Return to Top
1.3 Ceramic Biomaterials for Total Joint Replacement
There are three types of ceramics that are clinically relevant to contemporary total joint replacement, and these include alumina, zirconia, and alumina matrix composites. A fourth ceramic-like biomaterial, oxidized zirconium, is a ceramic-metal composite with a surface layer of zirconia. Each of these materials is discussed individually in the following subsections.
1.3.1 Alumina
Among ceramics, alumina has the longest history of successful use in hip replacements. The alumina in orthopedic implant components forms a stable crystalline structure known as alpha-alumina, which is characterized by a close-packed hexagonal configuration in which the Al and O atoms form strong ionic and covalent chemical bonds [7]. In this crystalline structure, the spatial arrangement of the alumina atoms creates a layer of negatively charged ions (O2- anions) at the surface that are the preferential sites for binding with positively charged ions (OH+ groups) present in water and joint fluid [7]. This also allows polar molecules (e.g. water or proteins) present at the surface of the component to establish a strong bond with the alumina surface [7]. Known as chemisorption, this mechanism explains why the wettability of the alumina surfaces is higher than that of metals, as depicted in Figure 1.3.
CeramTec GmbH (Plochingen, Germany) is the world’s largest supplier of medical grade alumina, which is distributed under the trade name of BIOLOX®forte. According to CeramTec, more than 4.5 million BIOLOX®forte femoral heads and 1.6 million acetabular inserts have been implanted on a worldwide basis as of December 2014 [12].
1.3.2 Zirconia
Zirconia ceramic was widely used in orthopedic applications between 1985 and 2001, until manufacturing difficulties at the world’s largest supplier resulted in its abrupt decline. Zirconia was initially chosen for commercialization due largely to its higher strength relative to alumina (Table 1.1). However, zirconia is a considerably more complex material than alumina. The reader is referred to several excellent reviews summarizing the properties of zirconia ceramic biomaterials [13-15].
Unlike alumina, zirconia is a “metastable” ceramic, consisting of monoclinic, tetragonal, and cubic phases [14]. Under a combination of temperature, humidity, and stress, zirconia can undergo a phase transformation from tetragonal to monoclinic, which results in a volume change [8, 14]. This phase transformation can have desirable consequences, such as the generation of a compressive stress field at the tip of a propagating crack, resulting in crack growth resistance. This material characteristic is referred to as “phase transformation toughening” [14]. However, the phase-induced volumetric transformation can also have disastrous consequences, if not properly controlled. For this reason, the phase transformation property of zirconia is typically stabilized with the addition of magnesia or yttria [8]. The most common type of zirconia used in orthopedics is termed Y-TZP, corresponding to yttria stabilized-tetragonal phase, polycrystalline zirconia [8]. The chemical composition of Y-TZP is about 5.1% yttria (Y2O3) and 93-94% zirconia (ZrO2) [14].
world’s largest supplier of medical grade stabilized zirconia ceramic. Between 1985 and 2001, the company reported that it has sold a total of 500,000 components fabricated from this material under the trade name of Prozyr® [16]. Starting in 2000, Desmarquest received reports of an unusually large number of fractures from components that were fabricated in early 1998, when the company implemented a change in manufacturing processes from use of a batch furnace to a continuous kiln or tunnel furnace [17]. These zirconia fractures were particularly troubling, because they involved components that complied with applicable technical standards. During August 2001, Desmarquest announced a voluntary recall of nine batches of femoral heads, all fabricated with a tunnel furnace during the late 1990s [17]. The recall had global implications, as the femoral heads were distributed to over 51 companies worldwide [17]. Recall announcements soon followed from national regulatory agencies. On September 13, 2001, the FDA warned that “surgeons should not implant artificial hips with the Saint-Gobain zirconia ceramic heads manufactured since the processing change in 1998” [17]. The company suspended distribution of zirconia ceramics for orthopedic applications in August 2001 [17]. After the recall, zirconia fell out of favor as a bulk orthopedic bearing biomaterial in the US and Europe.
The 2001 recall, and the ongoing research on zirconia ceramics, did not dampen the general enthusiasm for ceramic materials in orthopedics. Although alumina is currently the historic reference material for orthopedic applications, a newer and more complex alumina composite incorporating zirconia emerged in 2003 and has now been accepted as the current state-of-the-art biomaterial.
1.3.3 Alumina Matrix composite
Starting in 2003, a new alumina matrix composite material (BIOLOX®delta: CeramTec, Plochingen, Germany) has been available as a femoral head material [12]. This ceramic biomaterial is now broadly used across the orthopedic industry in both femoral heads and acetabular liners. According to the manufacturer, more than 3.6 million femoral heads and 1.5 million acetabular inserts have been implanted on a worldwide basis as of December 2014 [12]. As is the case with composite materials, the basic physical properties like stiffness, hardness, thermal conductivity, for example, are mainly determined by the dominant, alumina phase [7]. The primary advantage of this alumina matrix composite is its increased strength, fracture toughness, and wear resistance relative to alumina (Table 1.1) [18]. Additional details regarding alumina matrix composites can be found in recent reviews [6, 7].
BIOLOX®delta, the alumina matrix composite commercialized by CeramTec GmbH, consists of an alumina matrix (82% by volume) reinforced by zirconia phase (17 vol%) andstrontium hexaaluminate platelets (Figure 1.4). The zirconia phase and the hexaaluminate platelets are incorporated into the alumina matrix to provide toughening mechanisms, which explain the improved fracture resistance relative to alumina.
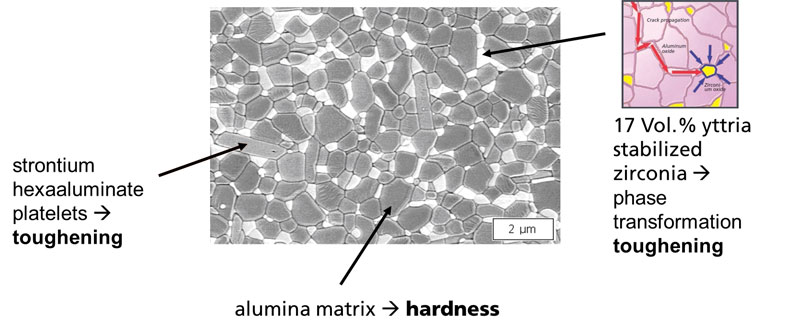
Figure 1.4. Chemical composition and microstructure of alumina matrix composite, BIOLOX® delta.
Alumina matrix composite ceramic is engineered to stabilize the tetragonal-to-monoclinic phase transformation in zirconia to improve the overall strength of the alumina matrix composite [19]. Clinical studies have now established the reliability of this new biomaterial in ceramic total hip articulations, as will be described in subsequent chapters of this website.
1.3.4 Oxidized Zirconium
Oxidized zirconium is a proprietary implant material of Smith & Nephew Orthopedics (Memphis, TN) marketed under the trade name OXINIUM [20]. OXINIUM material is fabricated by heating the zirconium alloy device in the presence of air, which converts the surface to a black zirconium oxide ceramic (~5 µm thick). In vivo phase transformation from metastable tetragonal to stable monoclinic is not an issue with oxidized zirconium, because its phase content is over 95% stable monoclinic. Clinically introduced for the knee in December 1997 and for the hip in October 2002, oxidized zirconium is intended only for hard-on-soft bearings both in total hip and total knee arthroplasty [21-24]. OXINIUM material is currently not used in hard-on-hard bearings.
^ Return to Top
1.4 The Origins of Ceramics in Hip Replacement
The application of ceramic materials in hip arthroplasty has its origins in Europe and Japan. The motivation to incorporate ceramics into artificial hips began in the late 1960s, when hip replacement surgery was still in its infancy. Although keenly interested by the potential of the procedure, surgeons were very concerned about the failures of these early hip replacements due to the excessive wear of the bearing materials used at the time, first fluorocarbons, and later polyethylene and polyacetal [7]. These early plastic bearing materials had poor clinical outcomes at the time, encouraging efforts to develop alternatives. Of these original plastic bearing materials, only polyethylene remains in clinical use today, and has itself been highly crosslinked and stabilized to overcome the historical problems that emerged from these early joint replacement designs.
Today, modern total hip replacement include one of three basic designs: (1) a metal ball head articulating against modern crosslinked polyethylene (M-PE) liner; (2) a ceramic ball head articulating against crosslinked polyethylene (C-PE); or (3) a ceramic ball head articulating against a ceramic liner (CoC). A fourth type of articulation, known as metal-on-metal (MOM), became widely used in the mid 2000s before concerns about metal debris and adverse local tissue reactions led to the rapid decline of all-metal articulations in the hip. Although no longer widely used, MOM bearings are mentioned here, briefly, for completeness.
With biological reactions to polyethylene wear, metal ions, and corrosion products still of clinical concern, it is instructive to reflect upon the circumstances that prompted pioneering researchers of the 1960s and 1970s to explore ceramics for total hip replacements. The historical sections that follow, adapted with permission from the more detailed review by Heros and colleagues [7], provide some context for how the main design features of today’s ceramic total hips came to be developed.
1.4.1 The Origins of Ceramic-on-Ceramic Hips from France (Late 1960s-1970s)
Motivated by the wear issues reported the earliest of plastic hip replacement components, Dr. Pierre Boutin, an orthopedic surgeon working in Pau, a town in southern France, decided to conduct extensive research on the use of alumina as an articulation material [7]. As an integral part of his research activities, Dr. Boutin began working with one of his patients who was the manager of the industrial ceramic manufacturer, CGE [7]. The result of their cooperation was the development of the first alumina ceramic on ceramic (CoC) articulation for THR [7]. The first implantation of this system was carried out in 1970 and reported in 1971 and 1972 [3, 4]. The THR system consisted of a stainless steel stem and an all-alumina head and cup with both components being cemented into bone. The alumina head was fixed to the femoral stem by the use of epoxy glue applied on a short cylindrical trunnion [7].
1.4.2 Early Innovations of Ceramic Hips from Germany (1970s)
In addition to the work of Boutin with Ceraver in France, research focused on the development of alumina bearings was taking place in Germany. It was during the 1970s that a “medical grade” alumina was developed by H. Dörre in the company Feldmühle (now CeramTec GmbH). This alumina material, branded under the trade name BIOLOX®, has since become a worldwide reference ceramic in orthopedics.
During the 1970s, several surgeons and scientists worked to develop ceramic devices [7], but in retrospect perhaps the most important of these early German ceramic-on-ceramic designs was the Autophor/Xenophor THR system developed by Mittelmeier [25]. The Autophor/Xenophor socket was designed as an uncemented component and had a macro-threaded external surface designed to achieve primary stability in the acetabular bed [7]. This cup design was intended to avoid loosening as a result of the failure of the cement mantle, a condition that was prevalent in the acetabular cups developed by Boutin [7]. Perhaps most noteworthy of the Autophor/Xenophor design was that it used an innovative taper locking system for the ball head and stem, an innovation that represents one of the most important contributions of the German scientists to the field of arthroplasty [7]. The taper connection—that was soon adopted in all the THR systems using ceramic ball heads—has proven its effectiveness in four decades of clinical use (Figure 1.5).

Figure 1.5. Ceramic to metal tapers
1.4.3 The Origins of Ceramic-on-Polyethylene Hips from Japan (1970s)
Japanese researchers also made significant contributions to the development of alumina for hip implants. Impressed by the early clinical fractures observed by Boutin’s initial ceramic-on-ceramic designs, researchers in Japan sought to improve the wear resistance of the historical polyethylene by articulation with a ceramic ball head. In 1977, Shikata in Japan introduced the concept of using alumina femoral heads with polyethylene acetabular components [5, 26]. Clinicians in the Kyoto region of Japan, working in collaboration with Kyocera (today known as Kyocera Medical, Osaka, Japan), developed the Bioceram prosthesis, consisting of polycrystalline alumina heads articulating with polyethylene sockets. Clinical research on the Bioceram ceramic-on-polyethylene (C-PE) prosthesis began in 1981, with favorable long-term follow-up [27, 28]. The Japanese innovation of combining ceramic on polyethylene in the hip also extended to developing the first C-PE and CoC designs of total knee replacement. Kyocera’s ceramic biomaterials, branded with trade name BIOCERAM® and developed exclusively for the Japanese orthopedic market, were never used outside of Japan. Nevertheless, the concept of C-PE bearing designs was quickly adopted internationally by surgeons, and today is one of the three most widely used types of hip replacement bearings in clinical use worldwide.
^ Return to Top
1.5 Summary
This primer has provided a technical overview of ceramic biomaterials, which will serve as a foundation for subsequent chapters on this website. We have discussed how the unique molecular structure of ceramic materials imparts many desirable qualities, including hardness, strength, wettability, and wear resistance, all of which are useful in a total hip replacement bearing material. We also reviewed how researchers have been working over the past 40 years to innovate on the composition of the ceramic materials themselves, leading to the development of first alumina, then zirconia, and most recently, alumina matrix composites, always in a quest to further improve the strength and inherent fracture resistance of the material. In addition, we provided a brief chronology of major innovations in the application of ceramics to hip replacement, including both historical CoC and C-PE designs and the incorporation of conical tapers in component design.
Although we touched on the topic of reliability of bioceramic materials only briefly in this chapter, this issue will be covered prominently in Chapter 2. In Chapter 3, we summarize the clinical performance of ceramic bearings in primary hip replacement, and in Chapter 4, we likewise discuss the clinical performance of ceramic bearings in revision surgery. Chapter 5 is devoted to the frontiers of ceramics for orthopedics, including applications in knee and shoulder arthroplasty. We invite you, the reader, to proceed to Chapter 2, where we will describe modern CoC and C-PE hip designs and discuss the reliability of ceramic hips as reported in the literature, especially around the topics of fracture and squeaking.
1.6 Acknowledgements
The author would like to thank Roland Huet, Exponent Inc., for many helpful discussions as well as technical and editorial feedback on this chapter.
^ Return to Top
1.7 References for Chapter 1
[1] Mendenhall S. Hospital resources and implant cost management–a 2013 update. Orthopedic Network News. 2014;25:9-15. [2] National Joint Registry for England and Wales. 11th Annual NJR Report. London: NJR; 2014. [3] Boutin P. [Alumina and its use in surgery of the hip. (Experimental study)]. Presse Med. 1971;79:639-40. [4] Boutin P. [Total arthroplasty of the hip by fritted aluminum prosthesis. Experimental study and 1st clinical applications]. Revue de chirurgie orthopedique et reparatrice de l'appareil moteur. 1972;58:229-46. [5] Shikata T, Oonishi H, Hashimato Y, al. e. Wear resistance of irradiated UHMW polyethylenes to Al2O3 ceramics in total hip prostheses. Transactions of the 3rd Annual Meeting of the Society for Biomaterials. 1977:118. [6] Kurtz SM, Kocagoz S, Arnholt C, Huet R, Ueno M, Walter WL. Advances in zirconia toughened alumina biomaterials for total joint replacement. J Mech Behav Biomed Mater. 2014;31:107-16. [7] Heros R, Pinconi C, Reinhardt C, Schneider N. Basic science of ceramics in total hip arthroplasty. The Adult Hip2015. [8] Willmann G. Ceramics for total hip replacement--what a surgeon should know. Orthopedics. 1998;21:173-7. [9] Willmann G. Ceramic femoral head retrieval data. Clin Orthop. 2000;379:22-8. [10] Merkert P. Next generation ceramic bearings. In: Zippel H, Dietrich M, editors. Bioceramics in Joint Arthroplasty, 8th Biolox Symposium Proceedings. Darmstadt: Steinkopff Verlag; 2003. p. 123-5. [11] Morlock M, Nassutt R, Wimmer MA, Schneider E. Influence of resting periods on friction in artificial hip joint articulations. In: Garino JP, Willmann G, editors. Bioceramics in Joint Arthroplasty, Proceedings of the 7th International BIOLOX Symposium. Stuttgart: Thieme; 2002. p. 6-20. [12] Heros R. Personal communication. CeramTec; 2015. [13] Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999;20:1-25. [14] Cales B. Zirconia as a sliding material: histologic, laboratory, and clinical data. Clin Orthop. 2000;379:94-112. [15] Clarke IC, Manaka M, Green DD, Williams P, Pezzotti G, Kim YH, Ries M, Sugano N, Sedel L, Delauney C, Nissan BB, Donaldson T, Gustafson GA. Current status of zirconia used in total hip implants. J Bone Joint Surg Am. 2003;85-A Suppl 4:73-84. [16] Information on breakages reported on Prozyr femoral heads: Key figures. http://wwwprozyrcom/PAGES_UK/Biomedical/figureshtm: St. Gobain, Desmarquest; 2002 (Accessed: April 28, 2003). [17] Information on breakages reported on Prozyr femoral heads: Key dates. http://wwwprozyrcom/PAGES_UK/Biomedical/historichtm: St. Gobain, Desmarquest; 2002 (Accessed: April 28, 2003). [18] Green DD, Williams PA, Donaldson TK, C. CI. BIOLOX-Forte vs. BIOLOX Delta under microseparation test mode in the USA. Transactions of the 51st Orthopedic Research Society. 2005;30:239. [19] Affatato S, Torrecillas R, Taddei P, Rocchi M, Fagnano C, Ciapetti G, Toni A. Advanced nanocomposite materials for orthopaedic applications. I. A long-term in vitro wear study of zirconia-toughened alumina. J Biomed Mater Res B Appl Biomater. 2006;78:76-82. [20] Sheth NP, Lementowski P, Hunter G, Garino JP. Clinical applications of oxidized zirconium. Journal of surgical orthopaedic advances. 2008;17:17-26. [21] Bourne RB, Barrack R, Rorabeck CH, Salehi A, Good V. Arthroplasty options for the young patient: Oxinium on cross-linked polyethylene. Clin Orthop Relat Res. 2005;441:159-67. [22] Good V, Ries M, Barrack RL, Widding K, Hunter G, Heuer D. Reduced wear with oxidized zirconium femoral heads. J Bone Joint Surg Am. 2003;85-A Suppl 4:105-10. [23] Li MG, Zhou ZK, Wood DJ, Rohrl SM, Ioppolo JL, Nivbrant B. Low wear with high-crosslinked polyethylene especially in combination with oxinium heads. A RSA evaluation. Transactions of the 52nd Orthopedic Research Society. 2006;31:643. [24] Ezzet KA, Hermida JC, Colwell CW, Jr., D'Lima DD. Oxidized zirconium femoral components reduce polyethylene wear in a knee wear simulator. Clin Orthop Relat Res. 2004:120-4. [25] Mittelmeier H. Report on the first decennium of clinical experience with a cementless ceramic total hip replacement. Acta orthopaedica Belgica. 1985;51:367-76. [26] Oonishi H, Wakitani S, Murata N, Saito M, Imoto K, Kim S, Matsuura M. Clinical experience with ceramics in total hip replacement. Clin Orthop Relat Res. 2000:77-84. [27] Saito M, Saito S, Ohzono K, Takaoka K, Ono K. Efficacy of alumina ceramic heads for cemented total hip arthroplasty. Clin Orthop Relat Res. 1992:171-7. [28] Sugano N, Nishii T, Nakata K, Masuhara K, Takaoka K. Polyethylene sockets and alumina ceramic heads in cemented total hip arthroplasty. A ten-year study. J Bone Joint Surg Br. 1995;77:548-56.



